UDI labelling obligation for dental products
For our customer, a manufacturer of reusable dental instruments, direct marking becomes mandatory. Find out what competitive advantages the customer was able to achieve with teltec systems' permanent laser marking.
Dental products are marked for various reasons with various content. The UDI labeling regulation applies to multi-use instruments that have to be reprocessed (e.g. autoclave) before each use. These include endodontic and periodontic instruments, various curettes and sinus lift instruments, implant drills, osteotomes, and condensers. Instruments like these also have to be marked with permanent CE signs or – as in the case of drills – with depth gauges.
Direct marking does not apply to sterile packed dental implants and screws.
However, for many reasons, it makes good sense to apply a direct laser mark on such dental products, and many manufacturers already do so: Brand logos, trademark signs, and the CE sign ensure protection against counterfeiting; laser marked depth gauges provide treatment safety, and reliably machine-readable 2D codes guarantee full traceability throughout the entire product lifecycle.
Challenges
Besides the various regulations – such as the UDI directive – hospital logistics related requirements are increasingly gaining importance. Clinics benefit from standardized, consistently marked and reliably traceable dental products. Manufacturers providing products in accordance with these demands enjoy distinct competitive advantages. Additionally, also functional markings such as depth gauges or optical markings for the purpose of the brand and counterfeit protection are required. High-quality standards, patient safety and traceability have to be ensured as well as an efficient and almost error-free production with as little scrap as possible.
Furthermore, next to the materials – ranging from Titanium nitride through stainless to Titan-oxidized surfaces as well as some plastic compounds –, it's the direct marking of the tiny devices that challenge the industry. The smallest markings have to be applied in minimal spaces with the highest resolution and contrast. All marks must be biocompatible and reliably readable. In the case of reusable reprocessed instruments, all marks have to
survive approximately ten to twenty autoclave cycles.
The Solution
Cost-effective dental device marking thanks to the integrated vision
FOBA's laser marking solutions are ideally suited for the reliable, permanent and economic identification of dental devices. Vision systems that are directly integrated into the scan head of the marking laser help to bring costly scrap down by 80%. Validation and verification steps before and immediately after marking directly in the laser system ensure lean and
stable marking processes and repeatable results. This is especially critical for manufacturers that have to meet strict quality standards: Marking contents are read, validated and verified immediately after marking while the device is still in the laser station.
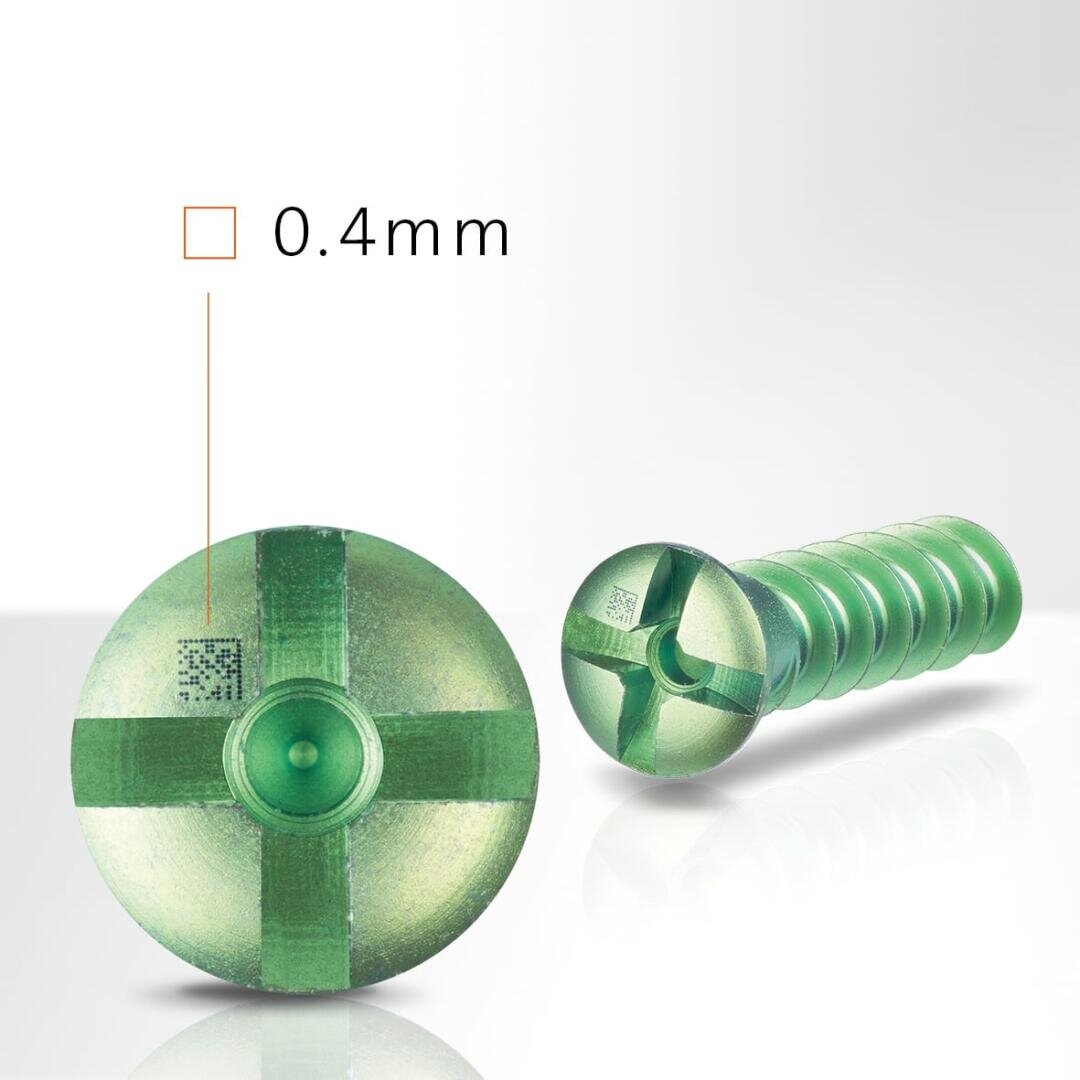
Tiny marks perfectly traceable
Our system and machine solutions are particularly suitable for marking even the tiniest micro 2D codes with high contrast. Despite their small size, even these small parts are reliably traceable even after repeated cleaning and processing.
FOBA marking lasers easily and flexibly integrate into production environments, and mark dental devices with all required content. Brand logos, trademarks, and graphics are applied as reliably, permanently and forgery-proof as serial and batch numbers, manufacturing dates and (UDI) codes (2D, etc.).